
The DNA damage response (DDR) and replication stress response (RSR) are sophisticated mechanisms that safeguard the integrity and stability of the genome. Mutations in DDR and RSR genes can cause cancer, premature aging, neurodegeneration, immunodeficiency, and developmental disorders. Many DDR/RSR factors are prominent drug targets.
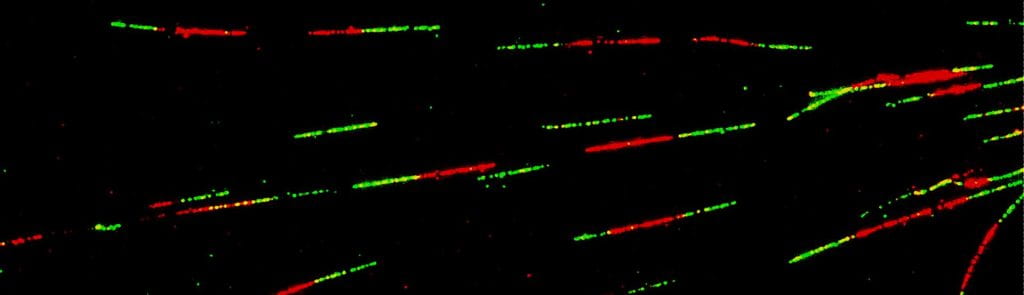
Our research has significantly advanced the understanding of the cellular response to DNA double-strand breaks (DSBs), in particular, the activation of the ATM-mediated checkpoint signaling and the process of DSB resection required for homology-mediated repair and the activation of the ATR checkpoint. Our more recent studies have led to the identification of a novel Ca2+-mediated signaling pathway that protects stalled replication forks under replication stress . This pathway involves the generation of cytosolic DNA fragments from replication stress, activation of the cGAS/STING axis, release of Ca2+ from the ER through the TRPV2 ion channel, and activation of the CaMKK2/AMPK/EXO1 protein phosphorylation cascade. Disruption of this pathway causes excessive replication fork processing, chromosomal instability, and reduced cell viability (Li et al., Mol Cell 2019; Li, Kong et al., Mol Cell 2023; Kong, Cheng et al., Nat Commun 2024. Also see accompanied Previews: Simoneau and Zou, Mol Cell 2019; Sohn, Mol Cell 2023).
Our ongoing research aims to further elucidate this new pathway, with a focus on the physical and functional interactions between cGAS/STING and TRPV2-mediated Ca2+ release, and the mechanisms of cytosolic DNA generation following replication stress or DNA damage. Additionally, we are exploring the roles of this pathway in other cellular processes, including the innate immune system, autophagy and senescence. These studies are laying the foundation for innovative therapeutic approaches targeting cancer, autoimmune diseases, chronic inflammation and neurodegenerative disorders.

Nonsense-mediated mRNA decay (NMD) is an RNA surveillance pathway that selectively degrades transcripts containing premature translation termination codons (PTCs), thereby preventing the synthesis of potentially harmful protein products. This pathway also serves as a gene regulatory mechanism that controls the expression levels of numerous physiological mRNAs. NMD is believed to modulate the clinical manifestations of various genetic diseases and cancer.
Our lab has developed multiple reporter systems that can effectively measure NMD activity in individual or groups of live human cells. Using these systems, we conducted a high-throughput drug screen and identified cardiac glycosides (CGs) such as ouabain and digitoxin as effective NMD inhibitors. Additionally, we carried out a genome-wide CRISRP/Cas9 screen and identified a number of NMD-promoting factors. With these reporter systems, we have also shown that NMD is inhibited by high levels of intracellular Ca2+ and persistent DNA damage in non-dividing cells (Nickless et al., Nat Med 2014; Nickless et al., JBC 2017). These findings may have important implications for the understanding of how Ca2+ regulates gene regulation and how the tumor microenvironment is affected by radiotherapy and chemotherapy.
Mutations in spliceosome factor genes like SF3B1, U2AF1, SRSF2 and ZRSR2 are common in hematological malignancies including myelodysplastic syndrome (MDS), chronic myelomonocytic leukemia (CMML) and acute myeloid leukemia (AML)). These mutations result in widespread missplicing and the generation of numerous nonsense mRNAs. Our recent studies have demonstrated that disrupting NMD with a small molecule inhibition of SMG1 (SMG1i) can selectively eliminate cancer cells harboring spliceosome mutations, thereby highlighting NMD as a potential therapeutic target for spliceosome mutant cancers (Cheruiyot, Li et al., Cancer Res 2021). In addition to the direct cell killing effects, inhibiting NMD inhibition may also promote immune clearance of cancer cells by increasing the production of cancer neoantigens. Our ongoing research is focused on evaluating the therapeutic potential of the NMD-targeting strategy in treating spliceosome mutant cancers, using both cell systems and animal models.